| EQUILIBRIUM CONSTANTS: Kc This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, Kc. It assumes that you are familiar with the concept of a dynamic equilibrium, and know what is meant by the terms "homogeneous" and "heterogeneous" as applied to chemical reactions. | |
We need to look at two different types of equilibria (homogeneous and heterogeneous) separately, because the equilibrium constants are defined differently.
A good example of a gaseous homogeneous equilibrium is the conversion of sulphur dioxide to sulphur trioxide at the heart of the Contact Process: A commonly used liquid example is the esterification reaction between an organic acid and an alcohol - for example: Writing an expression for Kc We are going to look at a general case with the equation: No state symbols have been given, but they will be all (g), or all (l), or all (aq) if the reaction was between substances in solution in water. If you allow this reaction to reach equilibrium and then measure the equilibrium concentrations of everything, you can combine these concentrations into an expression known as anequilibrium constant. The equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of A, B, C and D you started with. It is also unaffected by a change in pressure or whether or not you are using a catalyst. 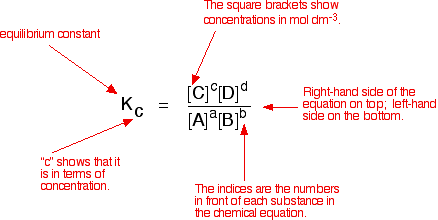 The indices (the powers that you have to raise the concentrations to - for example, squared or cubed or whatever) are just the numbers that appear in the equation. | |
| Some specific examples The esterification reaction equilibrium A typical equation might be: There is only one molecule of everything shown in the equation. That means that all the powers in the equilibrium constant expression are "1". You don't need to write those into the Kcexpression. 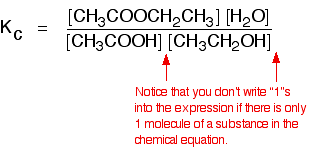 The equilibrium in the hydrolysis of esters This is the reverse of the last reaction: The Kc expression is: 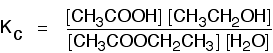 It is really important to write down the equilibrium reaction whenever you talk about an equilibrium constant. That is the only way that you can be sure that you have got the expression the right way up - with the right-hand substances on the top and the left-hand ones at the bottom. The Contact Process equilibrium You will remember that the equation for this is: This time the Kc expression will include some visible powers: 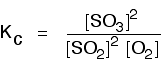 The Haber Process equilibrium The equation for this is: . . . and the Kc expression is: 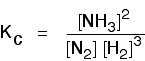 The equilibrium established if steam is in contact with red hot carbon. Here we have gases in contact with a solid. If you shake copper with silver nitrate solution, you get this equilibrium involving solids and aqueous ions: Writing an expression for Kc for a heterogeneous equilibrium The important difference this time is that you don't include any term for a solid in the equilibrium expression. Taking another look at the two examples above, and adding a third one: The equilibrium produced on heating carbon with steam Everything is exactly the same as before in the equilibrium constant expression, except that you leave out the solid carbon. 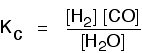 Both the copper on the left-hand side and the silver on the right are solids. Both are left out of the equilibrium constant expression. 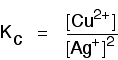 This equilibrium is only established if the calcium carbonate is heated in a closed system, preventing the carbon dioxide from escaping. The only thing in this equilibrium which isn't a solid is the carbon dioxide. That is all that is left in the equilibrium constant expression. | |
|
| |
Tuesday, November 4, 2014
EQUILIBRIUM CONSTANTS: Kc
Tuesday, October 28, 2014
Second Law of Thermodynamics
Second Law of Thermodynamics
The second law of thermodynamics is a general principle which places constraints upon the direction of heat transfer and the attainable efficiencies of heat engines. In so doing, it goes beyond the limitations imposed by the first law of thermodynamics. It's implications may be visualized in terms of the waterfall analogy.
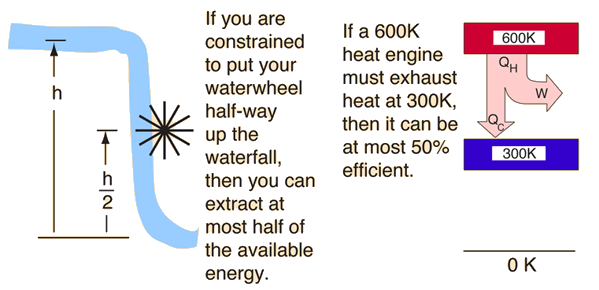
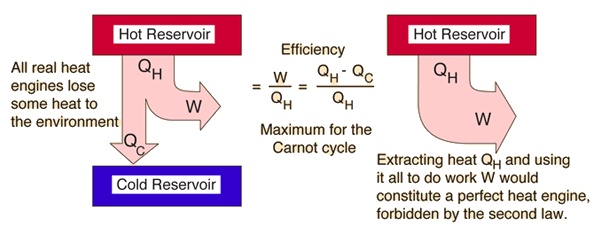
Second Law: Heat Engines
This is sometimes called the "first form" of the second law, and is referred to as the Kelvin-Planck statement of the second law.
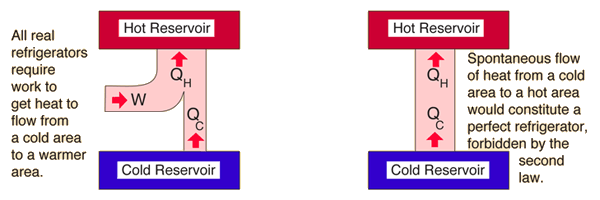
Second Law: Refrigerator
Second Law of Thermodynamics: It is not possible for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow. Energy will not flow spontaneously from a low temperature object to a higher temperature object. This precludes a perfect refrigerator. The statements about refrigerators apply to air conditioners and heat pumps, which embody the same principles.
This is the "second form" or Clausius statement of the second law.
Second Law: Entropy
| Entropy: | a state variable whose change is defined for a reversible process at T where Q is the heat absorbed. |  |
| Entropy: | a measure of the amount of energy which is unavailable to do work. | |
| Entropy: | a measure of the disorder of a system. | |
| Entropy: | a measure of the multiplicity of a system. |
First Law of Thermodynamics
The first law of thermodynamics is the application of the conservation of energy principle to heat and thermodynamic processes:
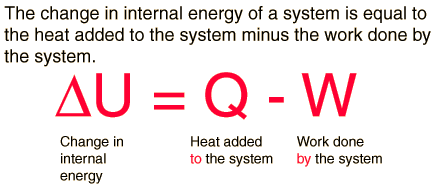
The first law makes use of the key concepts of internal energy, heat, andsystem work. It is used extensively in the discussion of heat engines. The standard unit for all these quantities would be the joule, although they are sometimes expressed in calories or BTU.
It is typical for chemistry texts to write the first law as ΔU=Q+W. It is the same law, of course - the thermodynamic expression of the conservation of energy principle. It is just that W is defined as the work done on the system instead of work done by the system. In the context of physics, the common scenario is one of adding heat to a volume of gas and using the expansion of that gas to do work, as in the pushing down of a piston in an internal combustion engine. In the context of chemical reactions and process, it may be more common to deal with situations where work is done on the system rather than by it.
Enthalpy
Four quantities called "thermodynamic potentials" are useful in the chemical thermodynamics of reactions and non-cyclic processes. They are internal energy, the enthalpy, the Helmholtz free energy and the Gibbs free energy. Enthalpy is defined by H = U + PV
where P and V are the pressure and volume, and U is internal energy. Enthalpy is then a precisely measurable state variable, since it is defined in terms of three other precisely definable state variables. It is somewhat parallel to the first law of thermodynamics for a constant pressure system Q = ΔU + PΔV since in this case Q=ΔH
It is a useful quantity for tracking chemical reactions. If as a result of an exothermic reaction some energy is released to a system, it has to show up in some measurable form in terms of the state variables. An increase in the enthalpy H = U + PV might be associated with an increase in internal energy which could be measured by calorimetry, or with work done by the system, or a combination of the two.
The internal energy U might be thought of as the energy required to create a system in the absence of changes in temperature or volume. But if the process changes the volume, as in a chemical reaction which produces a gaseous product, then work must be done to produce the change in volume. For a constant pressure process the work you must do to produce a volume change ΔV is PΔV. Then the term PV can be interpreted as the work you must do to "create room" for the system if you presume it started at zero volume.
System Work
When work is done by a thermodynamic system, it is ususlly a gas that is doing the work. The work done by a gas at constant pressure is: |  |
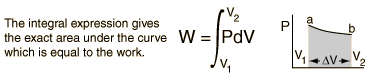
Monday, October 27, 2014
THE CONTACT PROCESS
| THE CONTACT PROCESS This page describes the Contact Process for the manufacture of sulphuric acid, and then goes on to explain the reasons for the conditions used in the process. It looks at the effect of proportions, temperature, pressure and catalyst on the composition of the equilibrium mixture, the rate of the reaction and the economics of the process. | |
|
A brief summary of the Contact Process The Contact Process:
Making the sulphur dioxide This can either be made by burning sulphur in an excess of air: . . . or by heating sulphide ores like pyrite in an excess of air: In either case, an excess of air is used so that the sulphur dioxide produced is already mixed with oxygen for the next stage. Converting the sulphur dioxide into sulphur trioxide This is a reversible reaction, and the formation of the sulphur trioxide is exothermic. A flow scheme for this part of the process looks like this: 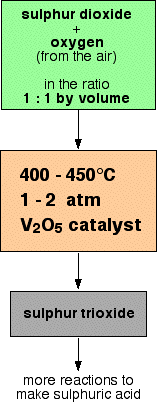 Converting the sulphur trioxide into sulphuric acid This can't be done by simply adding water to the sulphur trioxide - the reaction is so uncontrollable that it creates a fog of sulphuric acid. Instead, the sulphur trioxide is first dissolved in concentrated sulphuric acid: The product is known as fuming sulphuric acid or oleum. This can then be reacted safely with water to produce concentrated sulphuric acid - twice as much as you originally used to make the fuming sulphuric acid. Explaining the conditions The proportions of sulphur dioxide and oxygen The mixture of sulphur dioxide and oxygen going into the reactor is in equal proportions by volume. Avogadro's Law says that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. That means that the gases are going into the reactor in the ratio of 1 molecule of sulphur dioxide to 1 of oxygen. That is an excess of oxygen relative to the proportions demanded by the equation. According to Le Chatelier's Principle, Increasing the concentration of oxygen in the mixture causes the position of equilibrium to shift towards the right. Since the oxygen comes from the air, this is a very cheap way of increasing the conversion of sulphur dioxide into sulphur trioxide. Why not use an even higher proportion of oxygen? This is easy to see if you take an extreme case. Suppose you have a million molecules of oxygen to every molecule of sulphur dioxide. The equilibrium is going to be tipped very strongly towards sulphur trioxide - virtually every molecule of sulphur dioxide will be converted into sulphur trioxide. Great! But you aren't going to produce much sulphur trioxide every day. The vast majority of what you are passing over the catalyst is oxygen which has nothing to react with. By increasing the proportion of oxygen you can increase the percentage of the sulphur dioxide converted, but at the same time decrease the total amount of sulphur trioxide made each day. The 1 : 1 mixture turns out to give you the best possible overall yield of sulphur trioxide. The temperature Equilibrium considerations You need to shift the position of the equilibrium as far as possible to the right in order to produce the maximum possible amount of sulphur trioxide in the equilibrium mixture. The forward reaction (the production of sulphur trioxide) is exothermic. According to Le Chatelier's Principle, this will be favoured if you lower the temperature. The system will respond by moving the position of equilibrium to counteract this - in other words by producing more heat. In order to get as much sulphur trioxide as possible in the equilibrium mixture, you need as low a temperature as possible. However, 400 - 450°C isn't a low temperature! Rate considerations The lower the temperature you use, the slower the reaction becomes. A manufacturer is trying to produce as much sulphur trioxide as possible per day. It makes no sense to try to achieve an equilibrium mixture which contains a very high proportion of sulphur trioxide if it takes several years for the reaction to reach that equilibrium. You need the gases to reach equilibrium within the very short time that they will be in contact with the catalyst in the reactor. The compromise 400 - 450°C is a compromise temperature producing a fairly high proportion of sulphur trioxide in the equilibrium mixture, but in a very short time. The pressure Equilibrium considerations Notice that there are 3 molecules on the left-hand side of the equation, but only 2 on the right. According to Le Chatelier's Principle, if you increase the pressure the system will respond by favouring the reaction which produces fewer molecules. That will cause the pressure to fall again. In order to get as much sulphur trioxide as possible in the equilibrium mixture, you need as high a pressure as possible. High pressures also increase the rate of the reaction. However, the reaction is done at pressures close to atmospheric pressure! Economic considerations Even at these relatively low pressures, there is a 99.5% conversion of sulphur dioxide into sulphur trioxide. The very small improvement that you could achieve by increasing the pressure isn't worth the expense of producing those high pressures. The catalyst Equilibrium considerations The catalyst has no effect whatsoever on the position of the equilibrium. Adding a catalyst doesn't produce any greater percentage of sulphur trioxide in the equilibrium mixture. Its only function is to speed up the reaction. Rate considerations In the absence of a catalyst the reaction is so slow that virtually no reaction happens in any sensible time. The catalyst ensures that the reaction is fast enough for a dynamic equilibrium to be set up within the very short time that the gases are actually in the reactor. | |