This page describes the manufacture of ethanol by the direct hydration of ethene, and then goes on to explain the reasons for the conditions used in the process. It looks at the effect of proportions, temperature, pressure and catalyst on the composition of the equilibrium mixture and the rate of the reaction. | |
|
A brief summary of the manufacture of ethanolEthanol is manufactured by reacting ethene with steam. The reaction is reversible, and the formation of the ethanol is exothermic. A flow scheme for the reaction looks like this: 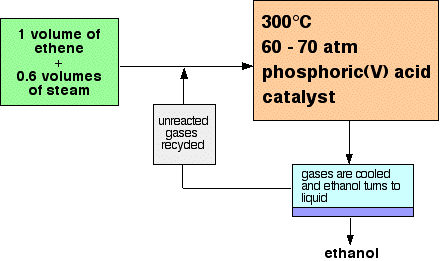 | |
|
Explaining the conditionsThe proportions of ethene and steam The equation shows that the ethene and steam react 1 : 1. In order to get this ratio, you would have to use equal volumes of the two gases. This is very surprising at first sight. Even if the reaction was one-way, you couldn't possibly convert all the ethene into ethanol. There isn't enough steam to react with it. The reason for this oddity lies with the nature of the catalyst. The catalyst is phosphoric(V) acid coated onto a solid silicon dioxide support. If you use too much steam, it dilutes the catalyst and can even wash it off the support, making it useless. The temperature Equilibrium considerations You need to shift the position of the equilibrium as far as possible to the right in order to produce the maximum possible amount of ethanol in the equilibrium mixture. The forward reaction (the production of ethanol) is exothermic. In order to get as much ethanol as possible in the equilibrium mixture, you need as low a temperature as possible. However, 300°C isn't particularly low. Rate considerations The lower the temperature you use, the slower the reaction becomes. A manufacturer is trying to produce as much ethanol as possible per day. It makes no sense to try to achieve an equilibrium mixture which contains a very high proportion of ethanol if it takes several years for the reaction to reach that equilibrium. You need the gases to reach equilibrium within the very short time that they will be in contact with the catalyst in the reactor. The compromise 300°C is a compromise temperature producing an acceptable proportion of ethanol in the equilibrium mixture, but in a very short time. Under these conditions, about 5% of the ethene reacts to give ethanol at each pass over the catalyst. The pressure Equilibrium considerations According to Le Chatelier's Principle, if you increase the pressure the system will respond by favouring the reaction which produces fewer molecules. That will cause the pressure to fall again. In order to get as much ethanol as possible in the equilibrium mixture, you need as high a pressure as possible. High pressures also increase the rate of the reaction. However, the pressure used isn't all that high. Problems with high pressures There are two quite separate problems in this case:
| |
| The catalyst Equilibrium considerations The catalyst has no effect whatsoever on the position of the equilibrium. Adding a catalyst doesn't produce any greater percentage of ethanol in the equilibrium mixture. Its only function is to speed up the reaction. Rate considerations In the absence of a catalyst the reaction is so slow that virtually no reaction happens in any sensible time. The catalyst ensures that the reaction is fast enough for a dynamic equilibrium to be set up within the very short time that the gases are actually in the reactor. | |