Second Law of Thermodynamics
The second law of thermodynamics is a general principle which places constraints upon the direction of heat transfer and the attainable efficiencies of heat engines. In so doing, it goes beyond the limitations imposed by the first law of thermodynamics. It's implications may be visualized in terms of the waterfall analogy.
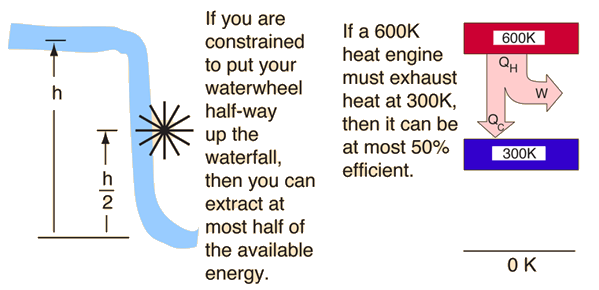
Second Law: Heat Engines
This is sometimes called the "first form" of the second law, and is referred to as the Kelvin-Planck statement of the second law.
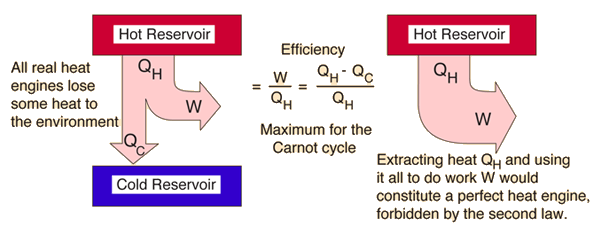
Second Law: Refrigerator
Second Law of Thermodynamics: It is not possible for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow. Energy will not flow spontaneously from a low temperature object to a higher temperature object. This precludes a perfect refrigerator. The statements about refrigerators apply to air conditioners and heat pumps, which embody the same principles.
This is the "second form" or Clausius statement of the second law.
Second Law: Entropy
| Entropy: | a state variable whose change is defined for a reversible process at T where Q is the heat absorbed. |  |
| Entropy: | a measure of the amount of energy which is unavailable to do work. | |
| Entropy: | a measure of the disorder of a system. | |
| Entropy: | a measure of the multiplicity of a system. |
No comments:
Post a Comment